H2S Scavenging: Using Triazine
H2S scavenging, or “gas sweetening,” is both a safety-critical and economic concern for ensuring trouble free upstream and downstream operations. This GATEKEEPER will discuss the use of triazine as a liquid H2S scavenger. Focal points include method of scavenging, application limits, treatment efficiency, production systems, downstream risks, as well as environmental impacts.
H2S Remediation Methods
Water injection for enhanced oil recovery always carries some risk of reservoir souring, whether produced water or sea water is injected. Various mitigation strategies can be employed to reduce this risk, including nitrate injection, low salinity (LoSal) sea water injection, and the use of sulfate removal units (SRUs). Various remediation strategies also exist for dealing with soured production, including catalyst scavenger beds, precipitator chemicals, amine units, and liquid scavenger chemicals. Selection of the most appropriate treatment strategy will depend on a variety of factors, including H2S concentration & gas volume, residence time, space & weight considerations, resources, process conditions, and CAPEX/OPEX. Mitigation, remediation, or a combination may be necessary to achieve export and safety specifications. Operating conditions will also influence the selection of treatment strategies and ultimate application locations. The most common methods have been highlighted below, but are also covered in more detail in the previous GATEKEEPER: Introduction to H2S Scavenging (GAT2004-2014.04).
Solid Scavengers
Solid scavengers are very effective in stripping H2S from gas streams down to trace levels; however, they require significant capital expenditure, and are often labor intensive during media change out. They generally have low OPEX, are predictable in their removal rates, usually do not require additional chemicals, and generally do not impact downstream processes or overboard water. As the spent waste of the non-regenerative solid options require removal or disposal, this can be impractical for offshore applications. The large footprint and limited capacity of regenerative solid options, combined with the production of a concentrated sour waste gas stream during regeneration, also make them less practical for offshore use.
Liquid Scavengers
Liquid scavengers, in general, take up less space and weight than solid scavengers, but are significantly less efficient at removing the H2S from the gas stream; the OPEX is significantly higher compared to solid scavengers. Liquid scavengers offer more options for retrofitting H2S scavenging to an existing facility.
Possible liquid scavenging options include, but are not limited to the following:
Triazine
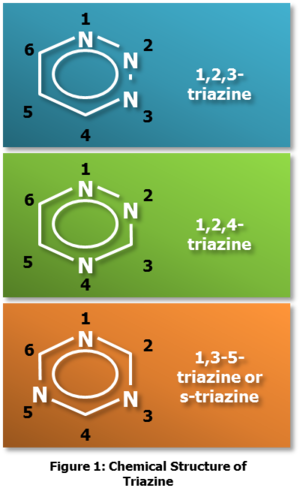
Triazine, the most commonly used liquid H2S scavenger, is a heterocyclic structure similar to cyclohexane, but with three carbon atoms replaced by nitrogen atoms. Oilfield terminology of triazine differs from the IUPAC convention, triazinane.
Three variations of triazine exist, based on the location of the substitution of nitrogen atoms, as shown in Figure 1. Further variations involving substitutions of the hydrogen atoms with other functional groups are used in various industries. This can be seen in Figure 2, with the substitutions occurring at any number of the “R” locations. Different substitutions result in different reactivity with H2S, changes in solubility of triazine, and changes in the solubility of the reactant products (the “R” groups). Consequently, triazine can be “tailored” to better suit the application or disposal considerations.
Application Methods
Direct Injection
In direct-injection applications, the triazine is sprayed directly into the gas or mixed fluid stream, usually with an atomizing quill. Removal rate is dependent upon the H2S dissolution into the triazine solution, rather than the reaction rate. As a result, gas flow rate, contact time, and misting size & distribution contribute to the final scavenger performance. This method is excellent for removing H2S when there is good annular-mist flow and sufficient time to react. Most suppliers recommend a minimum of 15 – 20 seconds of contact time with the product for best results. Typical efficiencies are lower due to the H2S dissolution into the product, but ~40% removal efficiency can reasonably be expected. In order for direct injection to be effective, careful consideration of injection location and product selection must be used.
Contactor Tower
In a contactor tower, the feed gas is bubbled through a tower filled with triazine. As the gas bubbles up through the liquid, gas dissolves into the triazine and H2S is removed. The limiting factors in this application are the surface area of the bubble, the concentration of the solution, and bubble path time (contact time). Finer bubbles give a better reaction rate, but they can produce unwanted foaming. This application is not appropriate for high gas flow rates. Contactor towers have much greater H2S removal efficiencies, up to 80%. As a result, far less chemical is used and a significant reduction in OPEX can be realized. However, the contactor tower and chemical storage take up significant space and weight, making them less practical for offshore application.
Reaction Process
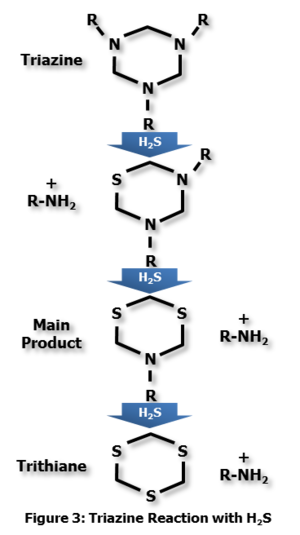
One mole of triazine reacts with two moles of H2S to form dithiazine, the main byproduct. An intermediate product is formed, but rarely seen. The reaction is shown in Figure 3. The R-groups that are released during the two-step reaction vary by the supplier, and can be tailored for solubility. Continued reaction can result in the formation of an insoluble trithiane product.
Downstream & Environmental Impacts
Reacted triazine byproducts are readily biodegradable and relatively non-toxic. Unreacted,excess triazine has very high aquatic toxicity and a tendency to form carbonate scale with produced water or sea water; this can result in emulsion stabilization, and increased overboard oil-in-water (OIW) content.
Unreacted triazine is also problematic for refineries as it impacts the desalting process and can cause accelerated corrosion within crude oil distillation units. It can also cause foaming in glycol and amine units and cause discoloration of glycol units. Unpleasant odor has also been reported with excess triazine usage, but some suppliers offer low-odor versions. Triazine itself is relatively safe to handle, but it can cause chemical burns upon contact.
Other Considerations
Triazine has been used successful around the globe by many operators and facilities. It has been used in various other applications where control of low-concentration H2S is vital, including scale remediation and reservoir stimulation. It is commonly used with sour shale gas production in the US.
Triazineis primarily used for removing low (<100 ppmv/mmscf) levels of H2S. It can be applied using a contact tower to increase (up to twice) the efficiency of H2S removal, but H2S levels >200 ppmv/mmscf will require the use of an amine-based sweetening unit. Triazine is also preferred in situations where the acid gas stream contains high levels of CO2 in addition to H2S. The triazine reacts preferentially with the H2S and the reaction is not inhibited by the CO2, avoiding unnecessary chemical consumption. It is also preferred where a concentrated sour waste gas streams cannot be accommodated or disposed.
Triazine is typically supplied in standard transportable totes, which can be unloaded into a larger storage tank on-site.
Conclusion
For offshore applications, direct injection of triazine is often the most economical and feasible method of H2S removal for gas and oil export lines; care needs to be taken to optimize the removal rates by selecting the optimal injection location and triazine-based product, keeping byproducts and disposal considerations in mind.
For onshore applications where space and weight are typically not an issue, contactor towers are far superior at H2S removal per volume of chemical used, and result in significantly lower OPEX. Ensuring contactor towers are appropriately sized and the bubble size is optimized for the gas production rate will make the maintenance and operation of the towers much easier and improve the realized OPEX savings over direct triazine injection.
For very high H2S levels (>200ppmv/mmscf), amine towers or solid media beds may have to be used for sufficient removal of H2S for the process and export considerations. Amine towers will be the topic of discussion in the next GATEKEEPER issue.