Epoxy Coating Selection & Failure Prevention
Offshore components often suffer from corrosion due to exposure to environments such as seawater, produced water, solvents, oxygen, CO2, H2S and other acids and abrasive particles. To protect equipment from degradation, coatings are applied to internal and external surfaces to provide electrical insulation, physical protection and corrosion and/or chemical resistance. Coatings can also provide thermal insulation, anti-slip, color coding, flame-retardant and anti-bio-fouling qualities to a given surface. This GATEKEEPER provides a general overview of the epoxy coatings used in offshore oil and gas service and discusses common causes of premature coating failure as well as factors affect coating quality.
EPOXY COATINGS
Epoxy coatings are commonly used in oilfield applications and are favored for their impact and abrasion resistance. Epoxy coatings are organic polymers which are created through chemical reactions between epoxy resins and co-reactants/hardeners/curatives. Epoxies are also thermosets, which means that once hardened or cured they cannot be melted down and reformed. During the process known as “curing”, terminal epoxy groups and mid-chain alcoholic hydroxyl groups allow for crosslinking to occur. Increasing the number of cross-links or cross-link density in a polymer decreases the mobility of individual polymer strands which causes the liquid polymer to solidify or “gel”.
Excessive heat will deteriorate chemical bonds within a thermoset and cause it to degrade, discolor, lose ductility, and/or undergo brittle failure. Epoxies are also vulnerable to photo-degradation and may chalk or discolor when exposed to UV rays.
EPOXY COATING SELECTION FACTORS
Typically, for a coating to be fit-for-service, the following factors must be considered:
Compatibility with substrate
Ability to accommodate stresses and impacts caused by transportation and installation
Ability to function under expected operating conditions such as temperature, pressure, and chemical environment
Electrical conductivity
Cathodic protection (CP) compatibility
UV resistance
Gas/liquid permeability
SURFACE PREPARATION
In addition to inappropriate coating selection, unexpected environmental changes or events, error during the application process and failure to provide proper maintenance can all cause the failure of an epoxy coating. It is estimated that 60% to 80% of all premature coating failures are caused by inadequate preparation of the substrate surface for application (1).
Improper surface preparation leaves behind contaminants and can cause adhesion failure as well as under-coating corrosion. For example, gas or liquid trapped underneath a coating will expand and cause blisters and delamination during rapid gas decompression. Similar failures also occur when gas or liquid penetrates a coating due to adhesion or permeation issues. Hand and power tool cleaning, blast cleaning and/or solvents are used to clean substrates. Blast cleaning is also used to develop the surface roughness needed to avoid adhesion failure. Depending on the substrate, an anti-oxidation chemical treatment may need to be applied soon after blasting. In addition to performing general blast cleaning, surface irregularities such as weld splatter, weld seams, metal slivers and sharp edges should be removed or smoothed as they are common causes of coating failures. Most end users require inspection of surface profile through visual examination and replica tape prior to proceeding with the application process.
APPLICATION
If an epoxy coating requires the substrate to be heated during the application process, the substrate should be uniformly preheated before application of coating. During the spray and cure process, ambient temperature and humidity should also be monitored and kept within acceptable range.
Most epoxies are applied via plural component sprays. There are some unique challenges and potential failure modes associated with this application method. Common issues that occur with spray gun use are clogged spray nozzles, spraying at too high a rate, moving the spray gun too rapidly, utilizing a coating material that is close to reaching its pot life, and off-ratio mixing in plural component sprays. Over application is also a common mistake made with epoxy coating. Though UV tolerance improves with dry film thickness, flexibility decreases and failure inducing residual stresses increase.
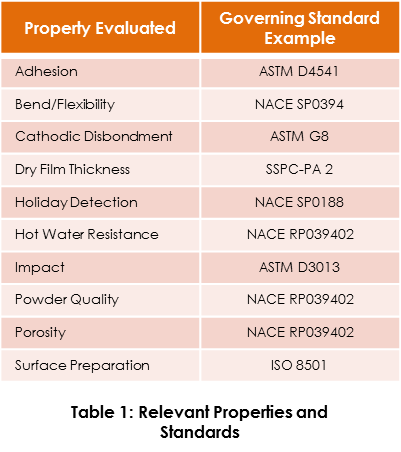
Quality control testing should be undertaken before and after application. Table 1 shows examples of properties that are commonly tested for quality assurance; however, this is not an exhaustive list. For each property, an example of a relevant governing standard is given. It should be noted that for comprehensive property evaluation tests, numerous standards (both public and proprietary), may be applicable.
FUSION BONDED EPOXY (FBE)
FBE coatings are applied in powder form to a pre-heated substrate via a spray gun or fluidized bed and then melted and cured by the heat from the elevated-temperature substrate.
FBE coatings are used as both standalone coatings and components of multilayer coatings. In multi-layer applications, the inner layer is most commonly used as corrosion protection and the outer layers function as abrasion, impact, temperature and/or UV resistance. Typically, FBE is heat resistant up to 230°F, though varieties with higher service temperatures do exist (3). FBE systems are also known for their low permeability to gases, particularly oxygen, chemical, and abrasion resistance (4).
FBE is particularly effective at corrosion protection because it partners well with cathodic protection (CP). It is thought that FBE’s relatively high permeability to water is what allows FBE to have an electrical resistance low enough to allow CP to work. FBE generally does not shield (divert current away from the underlying steel, thus rendering ineffective) CP when it experiences disbondment.
Even though the water that permeates FBE is relatively pure, if surface salt contaminants are not removed during the substrate cleaning process, they will react with any water that penetrates the coating and may cause the FBE to fail due to cathodic disbondment and blistering (5).
Other typical failure modes include loss of adhesion caused by inadequate surface preparation or application temperature, excessive holidays caused by overheating during the application process, deterioration or excessive water absorption due to overheating during operation, and chalking or disbondment due to long-term UV ray exposure.
EPOXY PHENOLIC
Unlike FBE, epoxy phenolics can come in liquid form. This is advantageous as liquid epoxy coatings do not require high application temperatures, though they may require elevated temperatures for curing. It should be noted that liquid epoxy coatings tend to have lower flexibility than fusion bonded epoxies, and consequently are susceptible to brittle failures.
During the application process the base and curing agent components are mixed and then applied as a liquid with tools such as paint brushes, rollers, floats, trowels or spray. Epoxy phenolics are cured at relatively high temperatures and may be applied in thicker coats and more varied curing conditions than many unmodified epoxies.
Two cure processes can be used; ambient cure, in which the phenolic and epoxy resins chemically react at room temperature, or baked cure in which the coating is baked at 350-400°F to accelerate cure reactions or activate a catalyst or curing agent in the coating (6).
Epoxy phenolics provide excellent chemical, solvent and temperature resistance and thus are most commonly used for immersion service, internal corrosion protection of downhole tubing, tank linings, high temperature oil and brine immersion service and other applications where severe chemical resistance is necessary, but a high degree of flexibility is not.
Advantages of epoxy phenolics include excellent adhesion properties, temperature resistance up to 400°F and resistance to solvents, chemicals, and abrasion (7). Disadvantages include decreased weatherability and flexibility, relatively slow air curing time and/or the necessity of heat curing at relatively high temperatures.
NOVOLACEPOXIES
Though often confused as being synonymous with phenolic epoxies, novolac epoxies are in fact a subcategory of phenolic epoxies.
Novolac epoxies have improved resistance to chemicals and high temperatures relative to other categories of epoxy phenolics such as those made with bisphenol A (BPA) and bisphenol F (BPF). The improved chemical resistance is attributed to novolac epoxy’s ability to achieve greater crosslink density due to a higher number of reaction sites on its chemical backbone. Improved heat resistance is caused by the presence of a large quantity of aromatic ring structures in its molecular structure. Novolac epoxies are heat resistant up to 356°F(8).
In general, novolac epoxies are also known for having greater resistance to oxidizing and nonoxidizing acids, and aliphatic and aromatic solvents than other epoxies. These qualities make novolac epoxies a suitable option for applications such as tank linings in contact with high temperature acidic crude oil.
CONCLUSION
Epoxy coatings require careful selection and qualification dependent upon the expected service environment. It is of equal importance to consider how quality assurance can be attained during the application process, as failure to do so may result in a variety of possible failure modes associated with the application phase.
REFERENCES
ASTM, 2009, Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers, D4541-09.
Prasanna R. Effect of Humidity on Surface Preparation and Coating Application. [Online]. Materials Performance. 2016, Feb 1. http://www.materialsperformance.com/articles/coating-linings/2016/02/effect-of-humidity-on-surface-preparation-and-coating-application
Zhou, W., Jeffers, T., Decker O.NACE, 2007. Properties of a Novel High Tg FBE Coating for High Temperature Service.
Khanna, A. S., 2008. High-Performance Organic Coatings. Cambridge, England: Woodhead Publishing.
Norsworthy, R. Fusion Bonded Epoxy- A Field Proven Fail Safe Coating System. NACE 2006.
Weldon, D. G., 2001. Failure Analysis of Paints and Coatings. Chichester: Wiley.
Phenolic Coatings. [Online] Metal Coatings. <http://www.metcoat.com/phenolic-coatings.htm>. Accessed 2016 Feb.
Murphy, J., 2001. Additives for Plastics Handbook. 2nd ed. New York, NY: Elsevier Science Inc.