Mercury Susceptibility In Process Facilities & How To Prevent Associated Failures
Mercury is commonly found in gas processing systems (midstream) and oil and gas fields throughout the world. Mercury is toxic to life and can have deleterious effects to several alloys commonly used in oil and gas production and refining industries. Based on an IPIECA data set, mercury levels gathered from 446 crude oil and condensate samples from different regions between 2007 to 2011 are summarized in Table 1 (1).
Table 1:
Regional Breakdown of Mercury Content in Crude and Condensate
The mechanisms that can lead to catastrophic failures of equipment in the oil and gas industry due to the presence of mercury are briefly presented in this GATEKEEPER. In addition, the detection, prevention, monitoring and maintenance of susceptible equipment, such as aluminum heat exchangers, is also considered.
FAILURE MECHANISMS: AMALGAM CORROSION
In oil and gas fields, the most common mercury failure is due to amalgam corrosion. Amalgam corrosion is defined as the process by which mercury forms liquid solutions with various metals, such as aluminum, silver, gold and zinc, among which it is aluminum that has mechanical significance. This type of corrosion is a combined effect of mercury and moisture on susceptible materials, and is a self-propagating process as long as a source of water is present.
The amalgamation depends on the wettability of mercury to the surface under aluminum oxide. This can further be impacted by thermal or mechanical stress, by chemical alterations, and by temperature. However, all aluminum alloys are susceptible to amalgam corrosion to some degree under a wide range of conditions. Amalgam corrosion can also develop in the form of pits if the mercury deposit is not continuous and liquid water is present.
FAILURE MECHANISMS: LIQUID METAL EMBRITTLEMENT
Liquid Metal Embrittlement (LME) is described as the loss of ductility and strength of a material due to diffusion of a low-melting-point metal into the exposed material structure. LME is characterized by the intergranular attack of liquid metal atoms leading to cracking. LME can be particularly destructive as, unlike many other cracking mechanisms, the magnitude of the applied stress leading to failure can be extremely low.
In the case of LME, the phenomenon described above is not limited to the presence of liquid mercury. For example, zinc embrittlement of austenitic stainless steel is also an example of LME. LME differs from amalgam corrosion in that it results in brittle fracture due to the intergranular attack, rather than the gross loss of material.
AFFECTED EQUIPMENT
As mentioned in the previous section, the most susceptible material to mercury attack in a processing facility, such as oil, gas and condensate gathering plants and liquefied natural gas (LNG) plants, is aluminum. This is most commonly used in such service in the construction of heat exchangers.
A particular concern for heat exchangers is at welds. For LME or amalgamation to occur, weld metal and mercury must be in intimate contact. This is due to the metallurgy of the welds, where the grain boundary chemistry and residual stress makes the area more susceptible to attack than the base metal. Hence, selection of weld materials, weld geometry and surface treatment all play an important role in mitigation. Field experience with aluminum heat exchangers suggests that LME is more likely to occur when the alloy contains magnesium as a strengthening element, due to the sensitization effect that this generates. This results from the segregation of the inter-metallic compound to grain boundaries during welding, thus increasing the susceptibility of weld area to mercury embrittlement (2).
The well documented Moomba Gas Plant explosion resulted in a gas release and fire due to LME (3). Investigation determined that this was due to the accumulation and attack of elemental mercury at the inlet nozzle of one of the cold boxes (main heat exchangers) shown in Figure 1. Further investigation also indicated that there were amalgam corrosion sites with pitting of the aluminum surfaces in several parts of the cold box.
DETECTION OF MERCURY
Current industry standards recommend maximum mercury concentration of 5 ppbv to ensure adequate equipment performance and protection, and complete removal of mercuryfor aluminum heat exchangers to the lowest routinely detectable level of 0.1 ppb.
Since mercury can bond to pipeline surface, it can take years for mercury to arrive at the process plant. Mercury readily bonds to most surfaces, but can also be highly mobile and found in any stream. In the field, significant variation of mercury concentrations have been observed from the same sources. This behavior can substantially complicate the associated field sampling and analysis.
In natural gas, mercury can be present as a metal in vapor phase or as an organo-metallic compound in liquid fractions. Common industry standards used for detection of mercury in produced fluids and process streams include the following:
ISO 6978-1: Natural gas - Determination of mercury - Part 1: Sampling of mercury by chemisorption on iodine.
ASTM 5954: Standard Test Method for Mercury Sampling and Measurement in Natural Gas by Atomic Absorption Spectroscopy.
ASTM 6350: Standard Test Method for Mercury Sampling and Analysis in Natural Gas by Atomic Fluorescence Spectroscopy.
ASTM D7623-10: Standard Test Method for Total Mercury in Crude Oil Using Combustion-Gold Amalgamation and Cold Vapor Atomic Absorption Method.
ASTM D7482: standard Practice for Sampling, Storage, and Handling of Hydrocarbons for Mercury Analysis.
PREVENTION & REMEDIATION
The amount of mercury reaching the processing facility should be monitored to prevent catastrophic failures and the resultant health, safety, environmental and asset integrity issues. In addition, the accumulation of mercury on internal surfaces of equipment over time should also be monitored.
To prevent the susceptibility of equipment and piping to mercury attacks, the following must be considered during the design phase of the project:
Materials of Construction
Process Design
MATERIALS OF CONSTRUCTION
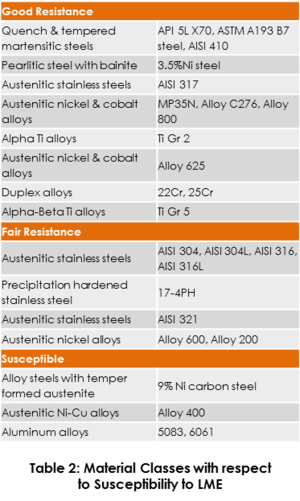
Since the metallurgy, welding, and surface treatment process has a great impact on the susceptibility of a material to mercury attack, GATE has undertaken a study of the extensive industry data published to-date in an effort to establish material classes with respect to the degree of susceptibility to LME; the generalized list is provided in Table 2.
Table 2 considers the results from multiple testing methods and the various testing conditions. Note that the costs of materials within the “good resistance” and “fair resistance” categories are significantly higher than that of aluminum. Therefore, the materials selection process must consider the application and equipment criticality in order to develop the most feasible solution.
PROCESS DESIGN
In addition to metallurgy, design of process flow to control moisture content, mercury content and temperature plays an important role in managing the risk of mercury attack.
By combining cooling, separation and chemical techniques, it is possible to control the water content in the inlet line to the gas plant heat exchangers to less than 0.5ppm. In order to achieve the required cooling, the incoming fluid needs to be cooled to within 10˚F to 20˚F of its hydrate temperature. Water removal can subsequently be achieved by a separator vessel, followed by absorption in molecular sieve beds.
An important issue related to molecular sieve beds is the downtime associated with the inefficient removal of water in the separator and high amounts of carry-over water received at the beds. To minimize downtime, separators andmolecular sieve beds need to be configured in series with contingent redundancy.
One option to consider for mercury removal is the use of silver-impregnated molecular sieve beds. This enables preferential amalgam formation between mercury and silver.
Another option for mercury removal is using non-regenerative metal sulfides at the inlet of the facility. Although this approach can produce the most benefit for high risk facilities by preventing mercury from entering the process system, it entails the use of larger vessels, and hence incurs a higher associated capital and operating cost.
CONCLUSION
As with many challenges in the oil and gas industries, the management and mitigation of mercury attack is often based on the associated balance of cost, incurred risk and the relative relationship between capital and operating costs over the life of a facility. This means that every project must address a different balance of factors, and that the development of a fit-for-purpose solution can be engineered given the right knowledge.
REFERENCES
IPIECA, Mercury Management in Petroleum Refining – Good Practice Guide, 2014.
Wilhelm, S.M., Risk Analysis for Operation of Aluminum Heat Exchangers Contaminated by Mercury, Wiley Interscience, April 2009.
https://abduh137.wordpress.com/2008/05/01/the-50-major-engineering-failures-1977-2007-part-4/