Bacterial Monitoring & Remediation In Pipelines
Bacteria inhabit the vast majority of oilfield water systems. These may either be attached to the pipe wall (i.e. sessile bacteria) or free floating through the system (i.e. planktonic bacteria). Planktonic bacteria do not directly contribute to the microbiologically induced corrosion (MIC) of pipeline systems; however, planktonic bacteria can attach to the pipe wall under the right conditions, becoming sessile bacteria. Consequently, there is some value in monitoring planktonic bacteria activity in a pipeline, although it is substantially less beneficial than monitoring the sessile population activity. The first line of defense against microbial proliferation in pipelines is to proactively sample and monitor the system for actively growing microorganisms. However, it is difficult to accurately predict where and when bacteria will grow in a pipeline system, and typical water analyses cannot always predict if microbial problems are to be expected in a new or existing system. In the oil and gas industry, NACE TM0194 is currently the preferred testing standard, and uses the Most Probable Number (MPN) method to quantify the bacteria in the sample. Results are obtained 14 to 28 days after the samples are inoculated, a major disadvantage if there is a real problem in the system. Other testing options, including genetic testing, can significantly decrease the time it takes to get results, increasing the likelihood of catching problems in the pipeline system earlier. If the results of the testing determine that there is a potential threat to the integrity of a pipeline system, then a mitigation and remediation strategy should be developed and implemented. This GATEKEEPER discusses the various bacteria testing options that are available, which can be used to validate MPN results, as well as the mitigation and remediation techniques that can be used to decrease the probability or severity of MIC to pipeline infrastructure.
OTHER BACTERIA TESTING OPTIONS
Many test methods are available to help determine the likelihood and extent of MIC related threats to the integrity of a pipeline system. Of these, the MPN test method has historically been the most common approach used; however, many other strategies are also available to support routine sampling or system troubleshooting. These include:
RapidChek®II
Second Generation Adenosine Triphosphate (ATP) Photometry
Quantitative Polymerase Chain Reaction (qPCR)
Denaturing Gradient Gel Electrophoresis (DGGE)
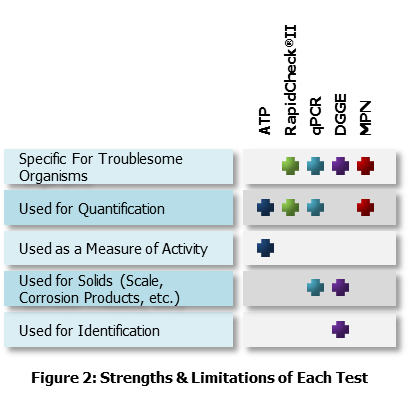
Best practice generally involves using a combination of test methods, as no single bacterial test is able to give conclusive results regarding bacterial activity in a pipeline system. Some tests are used to obtain numbers of total bacteria or different bacteria types, while others are used to determine the most prevalent bacteria types in the system. A combination of various testing techniques is often able to provide a more reliable distribution of the bacterial population in a given system.
ADENOSINE TRIPHOSPHATE (ATP) PHOTOMETRY
ATP Photometry allows the rapid quantification of all living organisms present in a sample. This test is not specific to bacteria but, since a majority of living organisms in the oilfield are bacteria, it can be a useful technique. The test does not identify the type of bacteria present, so other testing methods should be used in conjunction with ATP. The test takes as little as 30 minutes to complete and can be performed in the field provided there are trained personnel to perform the test.
ATP results indicate the relative abundance of metabolically-active total bacteria. A large number of dormant bacteria will yield a low ATP concentration, while a smaller number of metabolically active bacteria will yield a higher ATP concentration. Moreover, ATP values reflect the activity of the entire microbial population and cannot distinguish what amount of activity is due to specific types of bacteria.
RAPIDCHEK®II
RapidChek®II determines the approximate sulfate reducing bacteria (SRB) population on-site by quantification of the adenosine-5’-phosphosulfonate (APS)-Reductase enzyme through reaction with a chromagen. Visual analysis of the intensity of the resulting blue coloration in the test vial is used to estimate the SRB population. The APS-Reductase enzyme is common to all SRB, allowing for the measurement of the total SRB present in the sample to be obtained. Results are typically obtained within 30 minutes to 1 hour.
QUANTITATIVE POLYMERASE CHAIN REACTION (QPCR)
qPCR amplifies specific gene sequences from target organisms. During qPCR, genetic material is extracted from a sample and the numbers of copies of specific genes in the extract are quantified. Thus, unlike all the other methods, qPCR does not rely on visualization, viability, or separation of individual organisms. qPCR is also well suited for difficult samples, including solids, corrosion products or produced water. qPCR delivers the number of total bacteria, total Archaea, SRB and sulfate-reducing archaea (SRA). qPCR does not distinguish between live, inactive and dead cells, making it hard to characterize activity.
DENATURING GRADIENT GEL ELECTROPHORESIS (DGGE)
DGGE is a DNA-based technique which generates a genetic profile or fingerprint of the microbial population a pipeline system. Individual sequences or bands from this profile can be extracted and sequenced to identify the dominant members of the microbial population.
Applications of DGGE include assessment of changes in the population and bacteria types during biocide treatments. For example, if a biocide treatment is applied to kill a certain type of bacteria, a DGGE test can show what bacteria are left, if new bands have gained a foothold in the damaged biofilm, etc.
Generally a bacterial species must be present at 1% or more of the total population in order to produce a band that is clearly visible on a DGGE gel. Therefore, the DGGE bands allow for the detection of the most abundant species of bacteria present in a sample, where the intensity of the DGGE band is somewhat proportional to the relative abundance of the bacterial species in that sample.
BACTERIA MITIGATION AND REMEDIATION STRATEGY
A mitigation and remediation strategy should be developed in the event of any increase in microbial activity in a pipeline system. The mitigation and remediation strategy should include mechanical cleaning (maintenance pigging) and/or chemical treatment (biocides).
MECHANICAL CLEANING
Pigging removes paraffin that deposits along the pipeline wall and water that accumulates at low points in the pipeline. Removing most of the and paraffin deposits and water dropout minimizes environments that are favorable to bacterial growth in the pipeline systems. Maintenance pigs should be modified to include metallic brushes or scrapers when attempting to remove an established bacterial colony from a pipeline system.
CHEMICAL TREATMENT
Batch, semi-continuous or continuous biocide applications may be used. Batch treatment is the most practicable for pipeline applications; however, the standard industry practice for batch treatment would require multiple pigs in the pipeline at the same time. This is often undesirable due to the increased risk associated with a pig becoming stuck in the line.
It is recommended that the mitigation and remediation strategy include the use of biocides in a batch treatment if the bacteria sampling and monitoring program indicates high levels of bacterial activity in a pipeline system and the maintenance pigging frequency and pig aggressiveness cannot be increased.
CONCLUSION
At a minimum, ATP Photometry and RapidChek®II testing, combined with MPN testing, can provide a more reliable confirmation of bacteria in a pipeline system. ATP and RapidChek®II are bacterial test methods with short turnaround times for determining if bacteria or SRB, respectively, are present in the pipeline system. This allows for quick optimization of biocide treatments and will also serve as a check for MPN results. RapidChek®II may be substituted for ATP Photometry, but will only give results on the SRB population.
If there are discrepancies or lingering issues, genetic test methods such as qPCR and DGGE should be used to ensure that there are no problems with unculturable bacteria.
If high bacteria concentrations are detected and it is determined that the pipeline system contains a robust and prolific bacterial population, mechanical cleaning and/or chemical treatment will be required to mitigate MIC.
Regardless of the bacterial concentration levels, non-destructive testing (i.e. intelligent pigging) may still be required throughout the life of a pipeline system to directly assess metal losses, including those caused by MIC. However, the presence of a diversified microbial test program is generally sufficient to maintain risks at as low as reasonably practicable levels, until such time as a direct verification of the pipeline condition can be made.
REFERENCES
Chair Hartley H. Downs, Selection, Application, and Evaluation of Biocides in the Oil and Gas Industry, Task Group 075, Publication 31205, (Houston, TX: NACE International, February 2006).