Assessing Acid Flowback Integrity Risk
ACID FLOWBACK
Oil well stimulation is commonly undertaken using aqueous solutions of hydrochloric acid (HCl), hydrofluoric acid (HF), organic acids or mixture. The use of these acids will open new channels near the wellbore region for the oil and gas flow through and will result in increased production.
The acids used in downhole treatments are expected to induce severe corrosion attack on production tubing, downhole tools and casing, even though the anticipated contact times are kept short. To reduce the aggressive attack of the acid on tubing and casing materials, corrosion inhibitors are added to the acid solution. Even through inhibitors are known to provide some corrosion protection, they are usually effective only at high concentrations.
A significant issue with well stimulation is that the corrosion inhibitor is lost or is consumed to the reservoir prior to flowback. This can make the spent returns significantly more aggressive than the original formulation. Highly acidic pH values of around 1 are not uncommon. Recovery of the spent acid back through the well tubing may cause corrosion problems if the return fluids contain live acid (acid that has not reacted with mineral).
The carbon steel corrosion morphology caused by an unsuccessful acid flowback campaign is shown in Figure 1. This is mainly due to the low levels of corrosion inhibitors in the return fluids. In addition, surfactants, which are used to disperse the inhibitor in the acid, also have very high tendency to adsorb on formation surfaces. Therefore, it is vital to monitor and mitigate the risk of material integrity, including metallic and nonmetallic materials, during acid flowback.
CARBON STEEL CORROSION
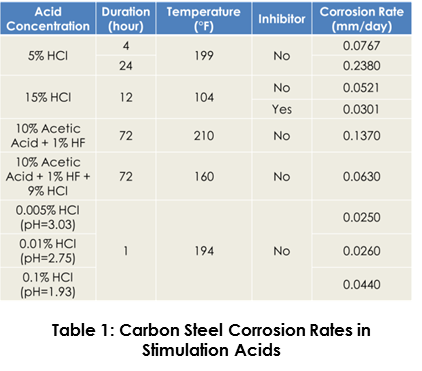
Carbon steel is extremely susceptible to corrosion when coming into contact with the spent acid, where the relatively high temperatures of the returns typically only serve to speed up the corrosion reaction. For instance, operational experience and laboratory testing indicate that a reasonable assumption for corrosion rates during acid flowback with HCl-based fluids is likely to be in the range of 50 mm/yr (0.137 mm/day), when the acid concentrations in the flowback fluid remains intact. The literature published regarding carbon steel corrosion in hydrochloric acid at different acid concentration and different temperatures is summarized in Table 1 (1-4). However, this is contrary to field experience where acids of similar and higher concentrations, albeit with inhibitors, have been successfully used. Therefore, the determination of a corollary flowback acid concentration that is more representative of what is in the flowback becomes necessary. This can enable the stakeholder to practically evaluate the integrity risk of the carbon steel components wetted by the flowback acids.
The generic GATE approach on evaluating the integrity risk of topsides carbon steel components is demonstrated below. Figure 2 shows example logs of pH vs. time for multiple flowbacks to a topsides facility. The overall trend is that the pH values of the acid flowback water fluctuated drastically during the initial 24 hours and then stabilized after 4 days. Where such data is available, it is conservative to assume the duration of the flowback acid impact on the topsides will be 4 days.
GATE uses statistical techniques and laboratory testing results to evaluate the probable accumulated damage to acid-wetted components based on topsides pH readings from prior or analog flowbacks. The minimum pH value on a daily basis measured at the flowback wells is used to determine the corresponding HCl mass concentration of approximated flowback water. Laboratory testing corrosion rates at corresponding low HCl mass concentration, as depicted in Table 1, can subsequently be used to calculate the total wall loss during the whole flowback. The general acceptance criteria is that if the accumulated wall loss for one acid flowback campaign is less than 0.1 mm, it is deemed to be very low risk as based on typical corrosion allowances, service lives and acid flowback frequencies.
CRAS CORROSION/CRACKING
Acids are not only corrosive towards carbon steel, but also attack corrosion resistant alloys (CRAs). For example, continuing with the example discussed above, well acidization and acid flowback with HCl, can be detrimental to austenitic stainless steels. HCl tends to preferentially attack the ferrite phase in austenitic stainless welds, resulting in stainless steel welds that can become severely degraded and ultimately fail.
The corrosion behavior of common oilfield CRAs in acids is summarized in Table 2 (5-7). These results indicate that all but 316 SS have negligible risk to continuous exposure to a relatively low concentration of HCl.
The exposure temperature also has an impact on the corrosion rate of CRAs. The corrosion resistance is generally considered good if the corrosion rate is less than 0.1 mm/yr or 0.00027 mm/d. Outokumpu has published data showing the maximum temperature where the general corrosion rate does not exceed 0.0003 mm/d at different HCl mass concentrations for different CRAs (8). It is indicated that 316 SS can withstand 0.10% HCl and 22 Cr DSS can tolerate 0.64% HCl when the exposure temperature is 80°C (176°F). Also, it is commonly accepted that the corrosion resistance of CRAs to HCl attack has the following ranking: 316 SS < 22Cr DSS < Alloy 625, based on the alloy composition and pitting resistance equivalent number (PREN). Alloy 625 is considered to be one of the most corrosion resistant alloys commercially available and has been shown to experience extremely low corrosion rates in many highly acidic solutions.
There is an elevated risk of stress corrosion cracking with acid flowback, especially at high temperature. Hence, it is important to avoid all potential isolated area where acid may become trapped and accelerate corrosion as the inhibitor deteriorates.
NONMETALLIC COMPATIBILITY
The correct selection and specification of sealing materials is a prerequisite to the effective operation and the prevention of expensive downtime. The common nonmetallics used in topsides include nitrile butadiene rubber (NBR), hydrogenated NBR (HNBR), Viton, Polytetrafluoroethylene (PTFE), Polyvinylidene fluoride (PVDF), Polyetherether-ketone (PEEK).
It is well known that NBR and HNBR will withstand only limited exposure to acids. NBR is typically considered to have poor chemical resistance compared to HNBR. However, HNBR is incompatible with high concentration acetic acid and HCl. Fluoroelastomers, such as Viton, are recommended as an upgrade for severe flowback conditions. Table 3 gives typical acceptance criteria for elastomeric and plastic-based materials, as based on a few key testing parameters.
PTFE is largely non-reactive, partly because of the strength of carbon-fluorine bonds, and so it is often used in containers and pipework for reactive and corrosive chemicals. Reinforced PTFE (R-PTFE) is the standard seat used in most non-cavity ball valves. 15% glass filled reinforced PTFE offers good chemical resistance to acetic acid and HCl. The only chemicals known to affect these carbon-fluorine bonds are alkali metals and highly reactive fluorinating agents, such as HF. Even so, it takes a relatively high concentration at elevated temperature before HF attacks PTFE.
PVDF is a special plastic used in applications requiring resistance to solvents, acids and bases. It has excellent resistance to high concentrations of HCl and acetic acid up to 93°C. Similarly, PEEK is widely regarded as a material with superb chemical resistance to acetic acid and HCl.
OTHER OPERATIONAL CAUTIONS
When receiving acid at a host facility, it is preferable to fully neutralize the spent acid with an alkali, or put the spent acid into tanks for disposal by a specialist contractor. If disposing of spent acid to a downstream asset is absolutely unavoidable, additional precautions need to be taken:
The disposal of the acid via process facilities and flowlines/pipelines can lead to major emulsion problems. Operation personnel should be ready to handle the high volumes of emulsions fluids.
Corrosion control chemicals formulated for use with the spent acid should be added at the recommended dosage rate before putting into the downstream flowline or export line.
After the spent acid is moved through the system, a pigging campaign should be undertaken as soon as practicable to sweep any residual acid out of the system.
It is recommended to increase the baseline corrosion inhibitor dosage rate for a period after the passage of the pig to ‘re-film’ the pipe wall as quickly as possible.
REFERENCES
Perdomo, J.J., et al, Corrosion Prevention during Acid Cleaning in Digesters and Evaporators. Corrosion, 2004, paper 04246.
Quraishi, M.A., et al, A Study of Some New Acidizing Inhibitors on Corrosion of N-80 Alloy in 15% Boiling Hydrochloric Acid. Corrosion Science Section, 2002, paper 317.
GATE Internal Data.
Tang, J., et al, Corrosion Behavior of Carbon Steel in Different Concentrations of HCl Solutions Containing H2S at 90°C. Corrosion Science, 52, 1715-1723, 2011.
http://www.outokumpu.com/SiteCollectionDocuments/Outokumpu-high-performance-austenitic-stainless-steel-data-sheet.pdf 2017.
http://www.outokumpu.com/sitecollectiondocuments/outokumpu-duplex-stainless-steel-data-sheet.pdf. 2017.
High-Performance Alloys for Resistance to Aqueous Corrosion. Special Metals Corporation. 2000.
http://www.outokumpu.com/SiteCollectionDocuments/Datasheet-316-316L–imperial–hpsa–outokumpu–en-americas.pdf